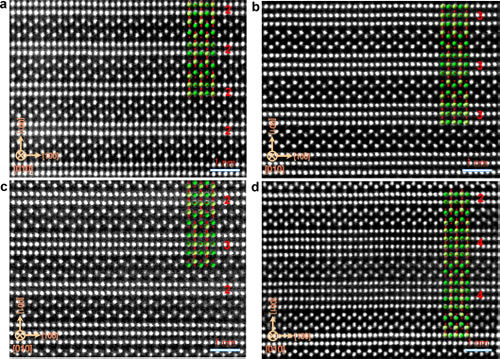
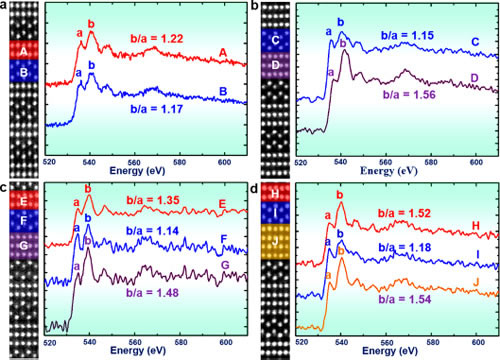
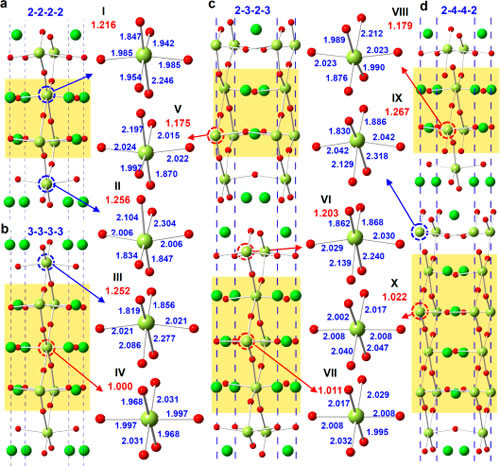
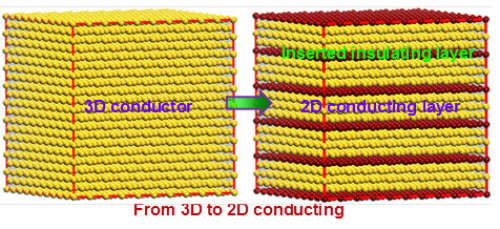
Recently, Professor Chen Chunlin, Ma Xiuling, researcher of Solid Atomic Image Research Department, Shenyang Institute of Materials Science, Chinese Academy of Sciences Institute of Metals, Chinese Academy of Sciences, Prof. Suez Laboratory, Nobel Laureate in Physics, Johannes Georg Bednorz, and Professor Yuichi Ikuhara, University of Tokyo, Japan In cooperation, the relationship between the conductive properties of the quasi-one-dimensional conductive material SrnNbnO3n+2 and its atomic electronic structure was established at the atomic scale, revealing the formation mechanism of its quasi-one-dimensional conductive properties. On this basis, the research idea of ​​preparing two-dimensional conductive material by inserting an insulating layer into a three-dimensional conductor is proposed. SrnNbnO3n+2 (ie SrNbOx) is a type of oxide having a layered perovskite structure, which can be obtained by introducing excess oxygen into SrNbO3. The structure and properties of these oxides are very sensitive to their oxygen content and distribution. The oxygen content determines the lamellar structure of the sheet thickness and conductivity characteristics. As early as more than 20 years ago, the research team led by Bednorz has realized that the quasi-one-dimensional conductivity of SrnNbnO3n+2 (electrical conductivity along the a-axis is much larger than that of other crystallographic orientations), and its mechanism of formation is tentatively discussed. However, it has long been a challenging problem to reveal the origin of its quasi-one-dimensional conductive properties at the atomic scale. Chen Chunlin et al. systematically studied the electrical properties of SrnNbnO3n+2 (including SrNbO3.4, SrNbO3.45, and SrNbO3.5 compounds) and their Nb ions using scanning transmission electron microscopy combined with first-principles theoretical calculations. The relationship between valence and NbO6 octahedral morphology. The results of electron energy loss spectra show that the conductivity of SrnNbnO3n+2 depends on the valence state of Nb ions: the region containing Nb4+ ions in the material has good conductivity, while the region containing Nb5+ ions is not conductive. Excess oxygen accumulates in the transitional region between the layers of SrnNbnO3n+2 (indented region in the text), forming an insulating layer, thereby making its conductive characteristics appear two-dimensional conductive characteristics. The results of first-principles calculations further show that the valence state of Nb ions is directly related to the morphology of its NbO6 octahedron: Nb4+ ions are always located at the center of the NbO6 octahedron, and Nb5+ ions are always far from the center. The electrical properties of the local region of the material in SrnNbnO3n+2 are determined by the morphology of its NbO6 octahedron. This study reveals the origin of the quasi-one-dimensional conductive properties in SrnNbnO3n+2 and provides ideas for the design and development of new quasi-one-dimensional conductive materials. At present, cooperative R&D for new quasi-one-dimensional conductive materials is underway. The research work was funded by the Frontier Science Key Research Project of the Chinese Academy of Sciences and the National Youth 1000 Personnel Program. Related results were published online at ACS Nano. Figure 1. HAADF image of the SrnNbnO3n+2 compound along the [010] ribbon axis. All compounds consist of alternately arranged chains and zigzag regions. (a) 2-2-2-2 type structure corresponding to n=4 (ieSrNbO3.5); (b) 3-3-3-3 type structure corresponding to n=5 (ieSrNbO3.4); (c ) 2-3-2-3 type structure corresponding to n=4.5 (ieSrNbO3.45); (d) 2-4-4-2 type structure corresponding to n=4,6,6,4(ieSrNbO3.4) ). Figure 2. EELS spectra of representative regions in the SrnNbnO3n+2 compound. (a) 2-2-2-2 type structure; (b) 3-3-3-3 type structure; (c) 2-3-2-3 type structure; (d) 2-4-4-2 type structure. The ratio b/a of the b-peak to the a-peak in the OK side reflects the valence of the Nb ion in this region. When the b/a value is large, Nb is +4 price, and vice versa is +5 price. The results of EELS indicate that the 3-3-3-3 type structure, 2-3-2-3 type structure and 2-4--4-2 type structure are two-dimensional conductors, and the 2-2-2-2 type structure is an insulator. Figure 3. Optimized structural model of SrnNbnO3n+2 compound, TDOS, and Nb-4dDOS. (a) 2-2-2-2 type structure; (b) 3-3-3-3 type structure; (c) 2-3-2-3 type structure; (d) 2-4-4-2 type structure. The results of DOS show that the 3-3-3-3 type structure, the 2-3-2-3 type structure, and the 2-4-4-2 type structure are two-dimensional conductors, and the 2-2-2-2 type structure is an insulator. Figure 4. Representative NbO6 octahedral morphology in the SrnNbnO3n+2 compound. (a) 2-2-2-2 type structure; (b) 3-3-3-3 type structure; (c) 2-3-2-3 type structure; (d) 2-4-4-2 type structure. The yellow background indicates the chain area. The ratio of bond lengths of Nb-O bonds in the zigzag region is larger, indicating that the Nb ion deviates from the central position of the oxygen octahedron. FIG. 5 shows a schematic diagram of preparing a two-dimensional conductive material: an insulating layer is introduced into the three-dimensional conductor so that it is converted from three-dimensional conductive to two-dimensional conductive. Fuonce-Lighting , https://www.fuoncelighting.com